Give The Procedures On How To Name Binary Covalent Compounds
Naming Binary Covalent Compounds 1 Write the name of the first nonmetal or metalloid. Modify the suffix of the second atom to have an ide ending.
 4 3 Covalent Compounds Formulas And Names Chemistry Libretexts
4 3 Covalent Compounds Formulas And Names Chemistry Libretexts
If youre still reading go to step 2.

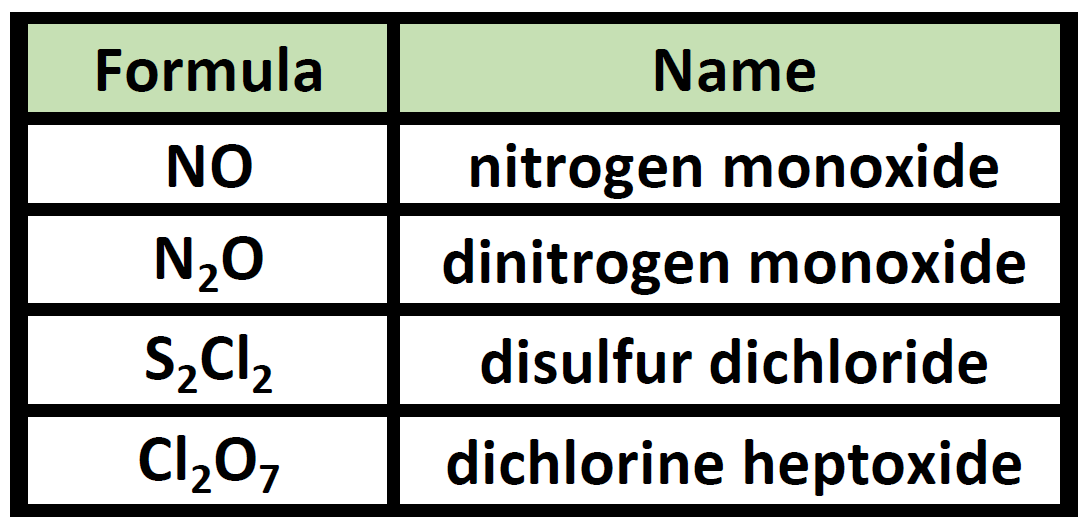
Give the procedures on how to name binary covalent compounds. Prefix name of nonmetal prefix root of name of nonmetalide eg. The prefix mono is never used for naming the first element of a compound. Rules for Binary Covalent Compounds.
The nomenclature of binary covalent compounds follows these rules. The first step in writing formulas when given the systematic name of a binary covalent compound is to recognize the name as representing a binary covalent compound. Explain how to write a formula for an ionic compound given the names of the metal and nonmetal or polyatomic ion in the compound.
To avoid awkward pronunciations the final o or a of the prefix is often dropped when the element name begins with a vowel. The second element is named by taking the stem of the element name and adding the suffix ide. The following table lists the most common prefixes for binary covalent compounds.
It will have one of the following general forms. Naming binary two-element covalent compounds is similar to naming simple ionic compounds. These examples show how the rules are applied for the covalent compounds formed by nitrogen and oxygen.
Mono- is only used as a prefix on the second element in the formula. Simple binary compounds consist of only a few atoms. Go back to the ionic naming tutorial and use those steps to solve the problem.
Number of Atoms in Compound Prefix on the Name of the Element. Attach the appropriate Greek prefix to the name of the first element. Systematic naming of these compounds follow the rules.
This is done by using Greek prefixes after the name of the element. Be able to name a molecular compound when given its formula. Common Prefixes for Binary Covalent Compounds Number of Atoms.
Numerical Prefixes for Naming Binary Covalent Compounds. If a binary compound is composed of two nonmetals it is a covalentmolecular compound. There are three rules in naming binary covalent compounds.
The first element in the formula is simply listed using the name of the element. Which of the following options correctly describe how to name a binary covalent compound. The final o or a of a prefix is often dropped when the element begins with a vowel.
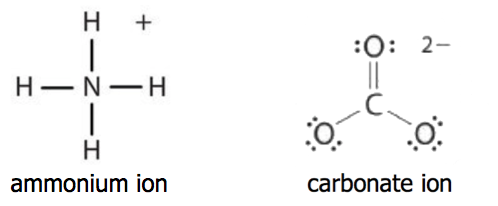
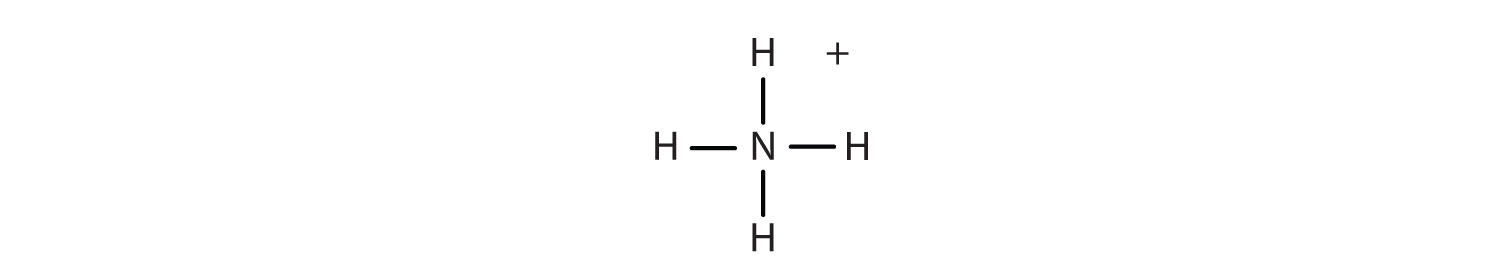
If the compound youre naming has a metal atom or the ammonium ion somewhere in it its an ionic compound and youre in the wrong tutorial. Make sure the compound youre trying to name is actually covalent. If there is more than one atom of this element include a greek prefix.
Lets review the Greek prefixes from one to ten. Name an ionic compound by the cation followed by the anion. A S4N2 B C120 C SFG D NO E IF- F SO3 Write Formulas For The Following Binary Compound Names.
Name Each Of The Following Binary Covalent Compounds. Prefixes are used in the names of binary compounds to indicate the number of atoms of each nonmetal present. Binary covalent compounds covalent compounds that contain only two elementsare named using a procedure similar to that used for simple ionic compounds but prefixes are added as needed to indicate the number of atoms of each kind.
Use the appropriate prefixes to name these simple compounds. If an atom in the compound starts with a vowel drop the vowel at the end of the prefix. Be able to write the formula for a molecular compound when given its name.
For binary compounds give the name of the first atom in the compound then the Greek prefix for the number of the second atom. First of all to name a covalent compound it helps to know what a covalent compound is. 2 Write the name of the second nonmetal or metalloid changing the ending to -ide.
Never use the prefix mono- in naming the first atom of a covalent compound. Although there are no ions in these compounds they are named in a similar manner to binary ionic compounds. A Chlorine Trifluoride B Carbon Tetraiodide D Phosphorus Pentabromide C Dinitrogen Pentoxide Name Each Of The Following As Binary Acids.
Binary covalent compounds are compounds made up of only two elements such as carbon dioxide. For example for CO the name will be carbon monoxide and the final o of mono is dropped. Know the common names for some simple molecular compounds such as methane CH 4 ammonia NH 3 phosphine PH 3 water H 2 O and hydrogen sulfide H 2 S.
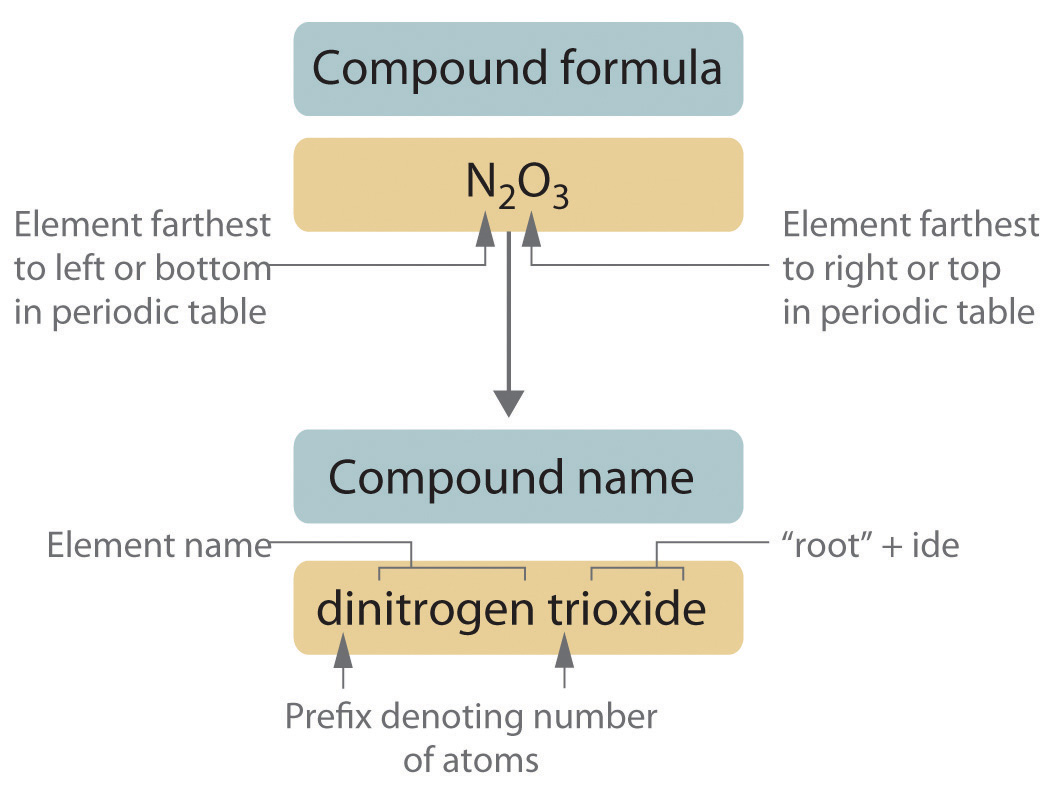
The procedure diagrammed in Figure 213 consists of the following steps. End the second atom with -ide. Select all that apply -the second element is named with its root and suffix -ide -the name of the first element remains unchanged except for the addition of a prefix where appropriate.
You may have used these in Geometry class. Write the symbol for the. The elements except for H are are written in order of increasing group number eg NO not ON The number of atoms of a given type is designated by a prefix such as di- tri- tetra- etc.
A covalent bond occurs between nonmetals only. When naming covalent compounds one must include the subscripts in the name. CO is called carbon monoxide.
351 Procedure for writing formulas for ionic compounds. Remember its only the final o or a. 1 6 2 7 3 8 4 9 5 10 The Rules for Naming.
In naming the compound CO all three rules are demonstrated. In a covalent bond one or more electrons are shared between two atoms. Binary covalent compoundsthat is covalent compounds that contain only two elementsare named using a procedure similar to that used to name simple ionic compounds but prefixes are added as needed to indicate the number of atoms of each kindThe.
 Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry
Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry
 Binary Ionic And Molecular Compounds Worksheet
Binary Ionic And Molecular Compounds Worksheet
Naming Binary Covalent Compounds Ppt Download
 An Introduction To Naming Binary Compounds Chemistry Ap Chemistry Science Chemistry Matter Science
An Introduction To Naming Binary Compounds Chemistry Ap Chemistry Science Chemistry Matter Science
 Eps To Naming Covalent Compounds 1 Identify If I Chegg Com
Eps To Naming Covalent Compounds 1 Identify If I Chegg Com
 How To Name Ionic Compounds And Covalent Compounds How To Wiki 89
How To Name Ionic Compounds And Covalent Compounds How To Wiki 89
 Naming Binary Covalent Compounds
Naming Binary Covalent Compounds
 2 12 Naming Chemical Compounds Chemistry Libretexts
2 12 Naming Chemical Compounds Chemistry Libretexts
2 7 Nomenclature Of Ionic Covalent And Acid Compounds Chemistry Libretexts
 Naming Covalent Compounds Nomenclature Rules
Naming Covalent Compounds Nomenclature Rules
 Covalent Compounds Formulas And Names
Covalent Compounds Formulas And Names
 Experiment 6 Nomenclature Of Inorganic Compounds
Experiment 6 Nomenclature Of Inorganic Compounds
 Solved Pre Lab For Experiment 7 2 Part A Inorganic Nome Chegg Com
Solved Pre Lab For Experiment 7 2 Part A Inorganic Nome Chegg Com
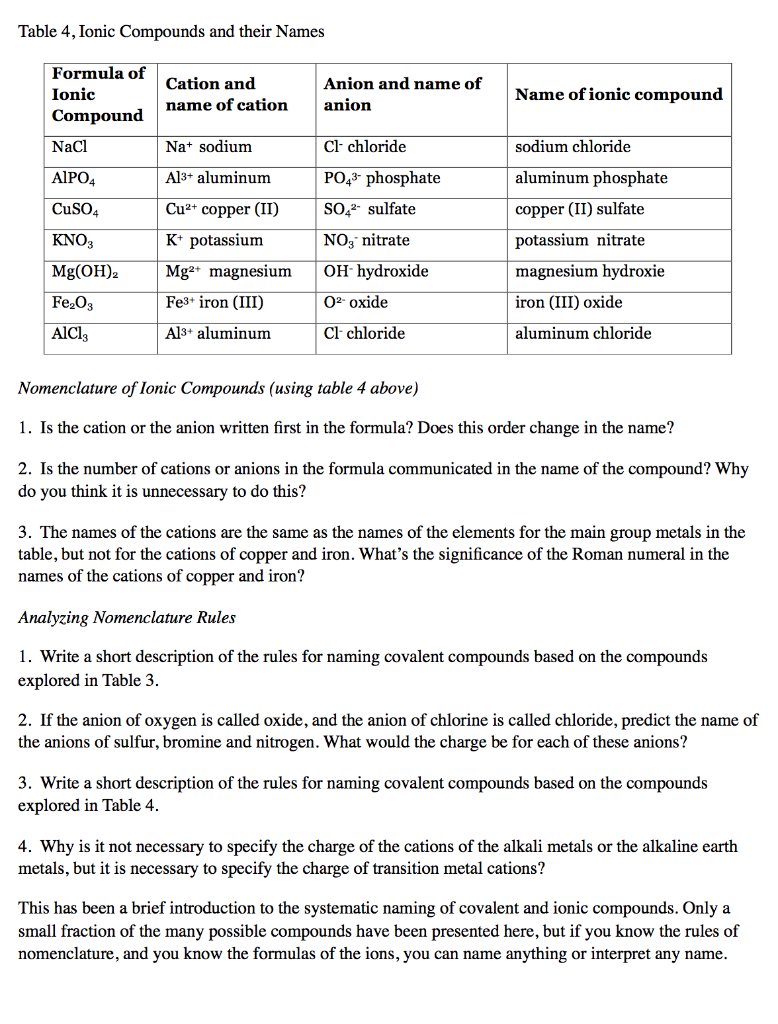 Solved Table 4 Ionic Compounds And Their Names Formula O Chegg Com
Solved Table 4 Ionic Compounds And Their Names Formula O Chegg Com
 How To Name Covalent Compounds Youtube
How To Name Covalent Compounds Youtube
 Naming Molecular Covalent Compounds
Naming Molecular Covalent Compounds
 Covalent And Ionic Naming Worksheet Teachers Pay Teachers
Covalent And Ionic Naming Worksheet Teachers Pay Teachers

Post a Comment for "Give The Procedures On How To Name Binary Covalent Compounds"